testing hardness of water titration|how to calculate water hardness : service Hardness water samples are determined by EDTA Titrimetric Method. Other than the total hardness of water there is “ Carbonate hardness ”. Carbonate hardness is also known as carbonate and bicarbonate alkalinity. In . スリル溢れるCall of Dutyのバトルを外でも楽しもう。最高 .
{plog:ftitle_list}
WEBWeebet - Sistema Completo de Apostas Esportivas e Cassino. Tenha sua própria casa de apostas esportivas & iGaming. Conheça o Weebet! Um sistema completo com recursos .
Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions. Complexometric titration is one of the best ways of measuring total water hardness. At pH around 10 EDTA easily reacts with both calcium and magnesium in the same .Water hardness can be readily determined by titration with the chelating agent EDTA (ethylenediaminetetraacetic acid). This reagent is a weak acid that can lose four protons on . Hardness water samples are determined by EDTA Titrimetric Method. Other than the total hardness of water there is “ Carbonate hardness ”. Carbonate hardness is also known as carbonate and bicarbonate alkalinity. In .
2340 HARDNESS* 2340 A. Introduction 1. Definition Originally, water hardness was understood to be a measure of the capacity of water to precipitate soap. Soap is precipitated chiefly by .There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with EDTA: Make a standard solution of EDTA. Use the standard EDTA solution to titrate the hard .You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium . This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.
A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and .titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. The strips contain EDTA andWater hardness can be readily determined by titration with the chelating agent EDTA . 50 mL of the water sample for each titration. As before, add 2 drops of indicator and 5 mL of pH 10 buffer before titrating. Carry out as many titrations as necessary to obtain three good trials. If the
A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. Hardness Test apparatus Requirements. Take 50 ml water sample; Conical flask 250 ml; Ammonia buffer solution: Dissolve 67.5 gm of ammonium chloride in 570 ml of ammonium hydroxide and dilute to one liter with deionised water. Eriochrome Black -T indicator: Dissolve 0.5 gm of EBT in 10 ml Methanol & makeup to 100 ml 0.02 N EDTA Solution: Take 3.723 gm of . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.
Determination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought . Half fill each tube with distilled water. The contents of all three test tubes should be the same colour. If any of the tubes is a different colour, it isn't clean enough. If .sample of bottled mineral water. 2. To compare experimental results with the concentrations of the metal ions claimed by the manufacturer. Introduction The ions involved in water hardness, i.e. Ca2+(aq) and Mg2+(aq), can be determined by titration with a chelating agent, ethylenediaminetetraacetic acid (EDTA), usually in the This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.Proceed to Sample Titration. Table 1 T7A Total Hard Method: Preprogrammed Parameters Electrode Parameter Electrode Type ISE-Titration ISE Type Calcium (Ca2+) . ASTM D1126, Standard Test Method for Hardness in Water. ASTM International, West Conshohocken, PA, USA. www.astm.org. 3. ISO 6059-1984, Water Quality – Determination of the sum of .

water test for hardness
Titration of calcium and magnesium (total hardness) in bottled and tap water by senior high school students from N. Alikarnassos High School in Crete, Greec. EDTA(Ethylene Diamine Tetra Acetic Acid) is a weak acid having molecular mass of 372.24 g . It has pH of 8 ; since it is a secondary standard solution , it pH increases with increase in temperature.Calcium Analysis by EDTA Titration One of the factors that establish the quality of a water supply is its degree of hardness. The hardness of water is defined in terms of its content of calcium and magnesium ions. Since an analysis does not distinguish between Ca2+ and Mg2+, and since most hardness is caused byMETHODS OF SAMPLING AND TEST (PHYSICAL AND CHEMICAL) FOR WATER AND WASTEWATER PART 21 HARDNESS ( Second Revision ) 1 SCOPE 1.1 This standard prescribes two methods for determination of total hardness, namely (a) Ethylenediamine tetraacetic acetate acid (EDTA) method, and (b) Method based on analytical data and
Titration methods. Hardness in water can be determined quickly by titration and the use of color indicators. By proper choice of pH, total hardness (Ca2+ and Mg2+) or the portion contributed by calcium and magnesium individually can be measured. . Calcium hardness titration. The test for calcium hardness is very similar to the total hardness .
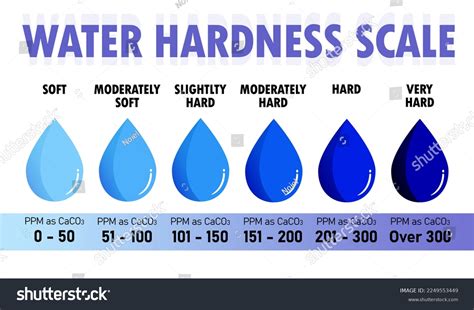
milligrams of CaCO3 per kg of water. The hardness of water may range from zero to hundreds of ppm, depending on the source. An excellent way to determine hardness of water is to perform a complexometric titration using a standard solution of .
Total Hardness.docx 1/4 . SI Analytics-Application report . Titration . Determination of total hardness in Water . Description . The determination of the total hardness in water is by titration with the sodium salt of done ethylenediaminetetraethanoic acid (EDTA), the detection is carried out with a Cu electrode and CuEDTA. Mg ion was used as standard to determine hardness of water by EDTA titration. The analysis showed cation exchange . [Show full abstract] capacity of zeolite X with molar ratio of Si/Al 1; 1,5 .
Proceed to Sample Titration. Table 1 T7A Total Hard Method: Preprogrammed Parameters Electrode Parameter Electrode Type ISE-Titration ISE Type Calcium (Ca2+) . ASTM D1126, Standard Test Method for Hardness in Water. ASTM International, West Conshohocken, PA, USA. www.astm.org. 3. ISO 6059-1984, Water Quality – Determination of the sum of .Determination of Water Hardness using Complexometric titration You will use EDTA complexometric titration to determine the hardness of a sample of water brought . Half fill each tube with distilled water. The contents of all three test tubes should be the same colour. If any of the tubes is a different colour, it isn't clean enough. If .

A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.
Determination of total hardness of water by complexometric titration of calcium and magnesium with EDTA at pH 10 using a Phototrode and Erio T color indicator. Products & Solutions. Laboratory Balances. Industrial Scales. Retail Weighing Scales. Rainin Pipettes and Tips. Analytical Instruments.
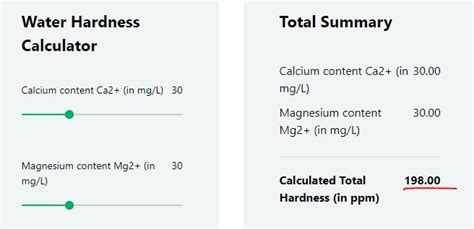
manganese may contribute to water hardness. Water hardness is often defined as the sum of the concentrations of Ca2+ and Mg2+ in water. “Hard” water typically contains high concentrations of Ca2+ and Mg2+, which react with the fatty acids in soap, causing them to precipitate. “Soft” water, such as rainwater or water that has passed . In most water samples the common cations EDTA would complex with would be Ca 2+, Mg 2+, and in some samples Al 3+, or iron species (Fe 2+, Fe 3+) Q3. The endpoint of a titration is determined using an indicator. What would be the general features of an indicator that could be used to determine the endpoint of a water hardness titration? 📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You can compare the color of the strip to the color chart, which will help you to determine your water hardness.
Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall .Titration. Hardness is commonly measured by colorimetric titration with an EDTA solution. A titration involves adding indicator and then titrant solution in small increments to a water sample until the sample changes color. . When the water hardness test strip is dipped in a water sample, a color develops on the strip and the strip is matched .
the 25 mL of the “hard water” sample should be measured with a 25-mL transfer pipet and the EDTA solution should be added from a buret. Preparation of Solutions Hard water sample. A hard water sample that mimics very hard water with approximately 1,000 ppm Ca2+ ion may be prepared by creating a slurry of 0.25 g of anhydrous CaCO
with lawn mower compression test
water hardness test method
web24 de out. de 2023 · Ninguém acertou as dezenas do concurso 2648 da Mega-Sena e prêmio acumulado está estimado em R$ 60 milhões; veja números sorteados.
testing hardness of water titration|how to calculate water hardness